Etavopivat and Defining Deformability
PKR Activators for SCD
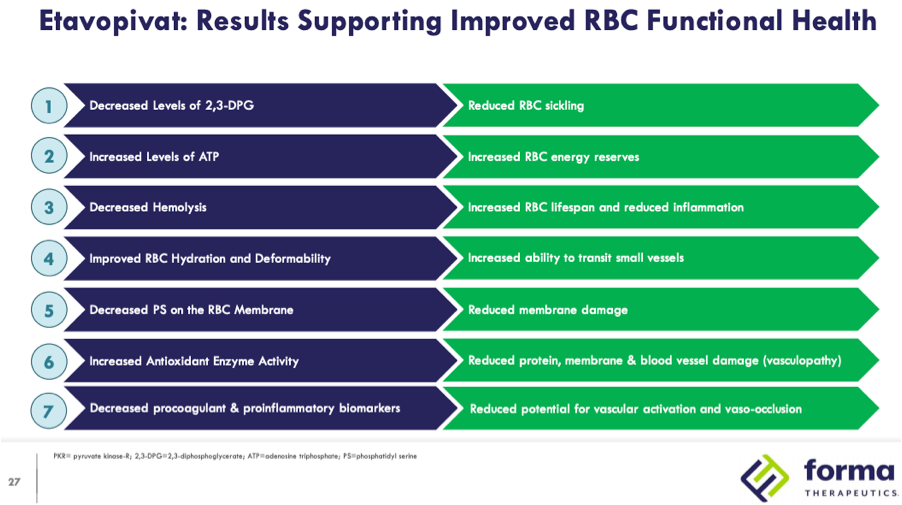
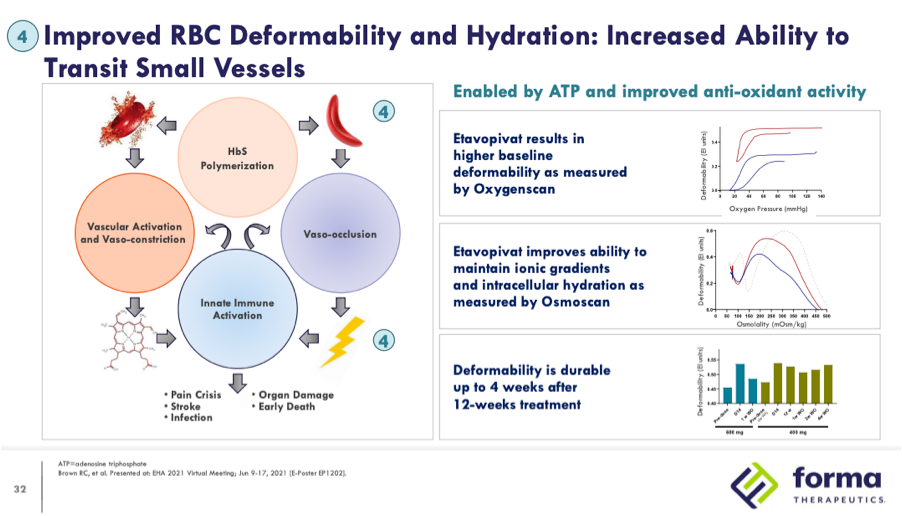
Following the approvals of Adakveo and Oxbryta for the treatment of sickle cell disease (SCD) in November 2019 there are a number of clinical candidates in development vying to be the next therapy approved in this indication. Among the more advanced candidates are two pyruvate kinase-R (PKR) activators, mitapivat (AG-348), developed by Agios Pharmaceuticals, and etavopivat (FT-4202), developed by Forma Therapeutics. Forma recently initiated HIBISCUS, a Phase 2/3 study in March of 2021 with an estimated primary completion in December 2025 and Agios plans to initiate a Phase 2/3 study by year-end 2021 to support approval of mitapivat in 2026. Both companies contend that there is still a significant unmet need in SCD that can be addressed by PKR activators through their multi-modal mechanism of action. Data from Phase 1 studies of mitapivat and etavopivat demonstrated proof of concept of the ability of PKR activators to decrease 2,3-diphosphoglycerate (2,3-DPG) and increase adenosine triphospate (ATP.) PKR activators are thought to confer a number of benefits summarized in the below slide from a Forma investor presentation:
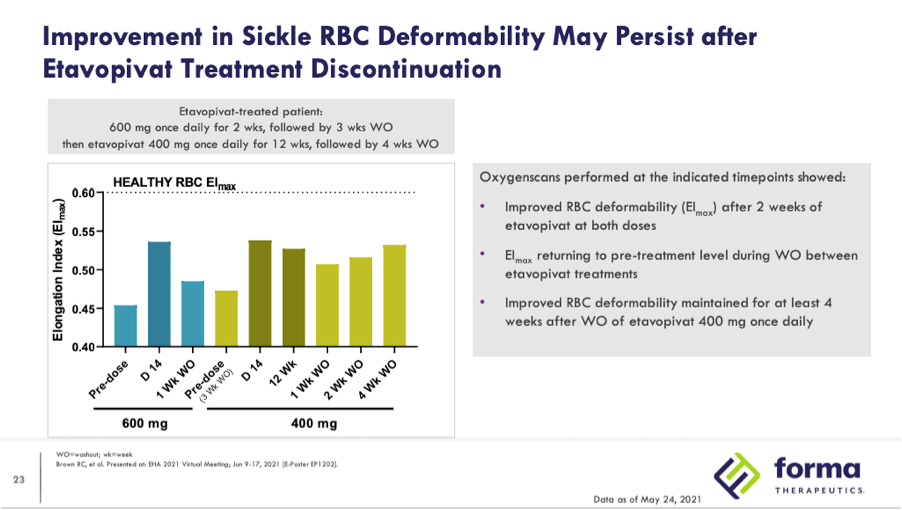
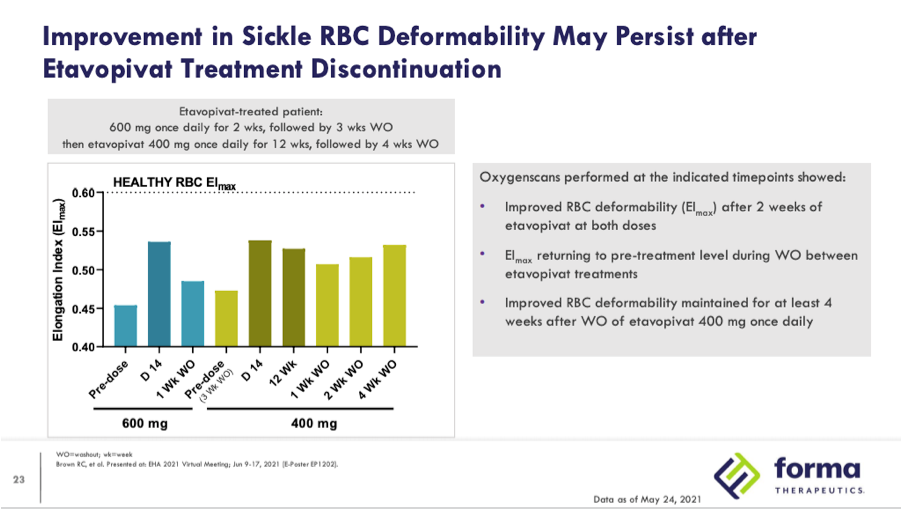
While the data demonstrates clear decreases in 2,3-DPG and increases in ATP, skeptics of this approach fear that PKR activators may actually increase vasco-occlusive crises (VOCs), pointing to safety data from the initial clinical results and theorizing about what will be seen in larger trials of longer duration. In Phase 1 data presented by Agios 12 patients were enrolled with 9 given a dose of 5mg BID, 20mg BID, and 50mg BID for two week’s duration each before undergoing a 12-day taper after completing dosing. The other 3 patients were escalated to a 100mg BID dose prior to commencing a 15-day taper. The company noted that one VOC occurred during drug taper and two other VOCs occurred during the 28-day safety follow-up post drug exposure. Despite the first VOC being attributed as possibly related to the drug the other two were not attributed to mitapivat. While two of the VOCs occurred during the 28-day safety follow up post-taper, data from Forma presented at EHA 2021 suggests that the effects of PKR activators may persist for at least four weeks after washout:
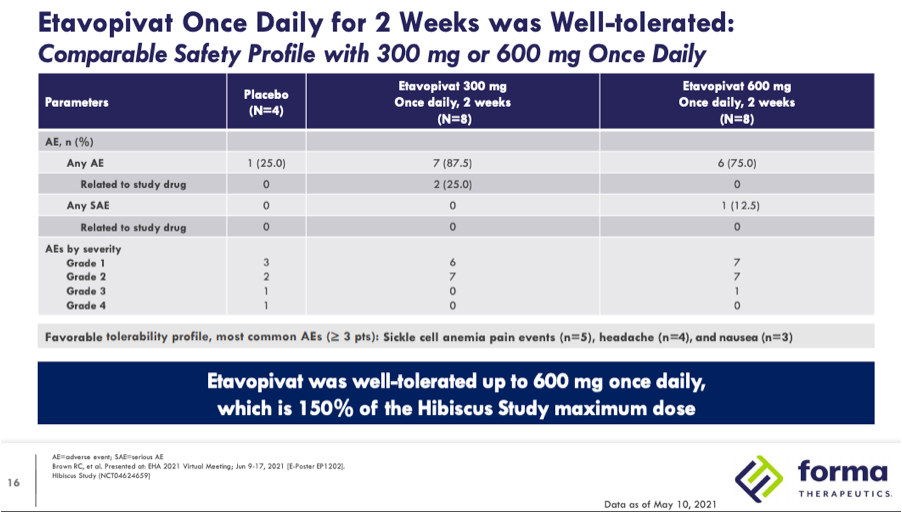
Forma presented blinded Phase 1 data at ASH 2020 from 9 SCD patients enrolled in their 300mg QD or placebo 14-day cohort. In that study there were six grade 2 treatment-related adverse events that included 3 uncomplicated sickle cell pain events in 2 patients that were all considered unrelated to treatment and consistent with each patient’s SCD pain history (the company defines pain events as events that only require home treatment as opposed to hospitalization as with VOCs.) At EHA 2021 the company presented un-blinded Phase 1 data that also included data from a 10 patient 600mg QD or placebo for 2 weeks cohort as well as a 10 patient 400mg QD for 12 weeks open-label extension (OLE.) Safety data from the 300mg and 600mg QD cohorts below does not break out the adverse events beyond their severity for placebo and each dose but does note that sickle cell anemia pain events were one of the most common adverse events, occurring in 5 patients.
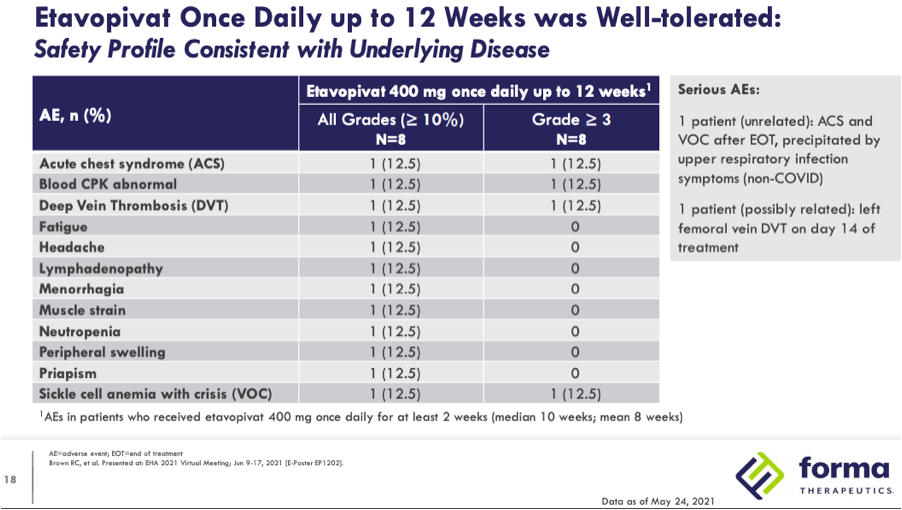
In the 400mg QD for 12 weeks OLE one patient had a VOC and acute chest syndrome (ACS) that was precipitated by upper respiratory infection symptoms and deemed unrelated. There was also one patient with deep vein thrombosis during treatment that was considered possibly related.
Taking into account the small number of patients treated, short length of the duration of treatment and lack of VOC history in these patients it is difficult to draw any strong conclusions about rate of VOCs and their attribution. Despite this it is worth examining the other data presented to date that may help predict whether PKR activators can be reasonably expected to show a benefit in a placebo-controlled pivotal trial.
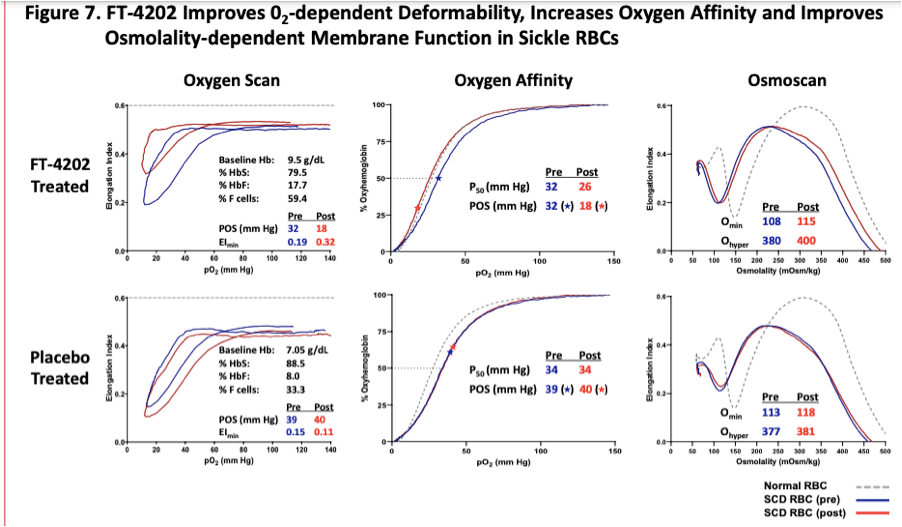
Returning to Forma’s EHA 2021 presentation, the fourth highlighted result of treatment, improved red blood cell (RBC) hydration and deformability, is proposed to increase the ability of RBCs to transit small vessels.
Throughout their development of etavopivat Forma has consistently highlighted data from oxygenscan to measure improvements in deformability of RBCs.
Oxygenscan
Oxygenscan is a recently developed functionality of the Lorrca Platform that allows for measuring RBC deformability expressed as Elongation Index (EI) utilizing three key parameters:
· EImax: RBC deformability at normoxia
· EImin: RBC deformability upon deoxygenation
· Point of sickling (PoS): the point at which a >5% decrease in EI is observed during deoxygenation, reflecting the patient-specific pO2 at which sickling begins
Utilizing RBCs from two cohorts of SCD patients in Europe (n=62) and the US (n=97) this study looked at the relationship between VOCs and the three parameters outlined above. There was a significant difference in PoS and EImin between patients who had a VOC in the last 2 years compared to those without. In contrast, EImax did not show a significant difference between those with and without VOCs. The researchers also looked at the effects of transfusion and hydroxyurea on those three parameters with both treatments showing improvement on all 3 compared to baseline.
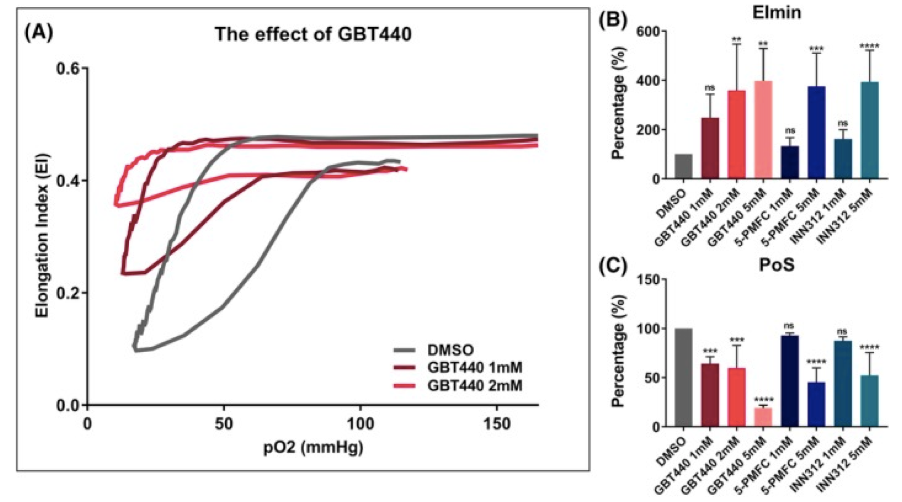
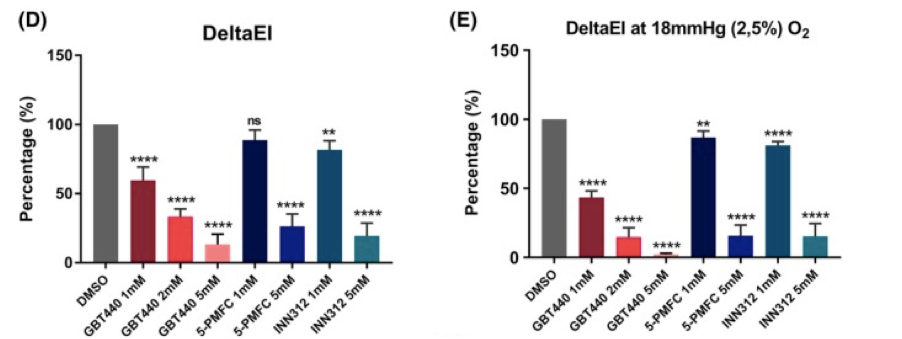
In this paper looking at in vitro treatment with antisickling agents GBT440 (Oxbryta), 5-PFMC and INN312 all show a clear effect on DeltaEI with improvements on EImin.
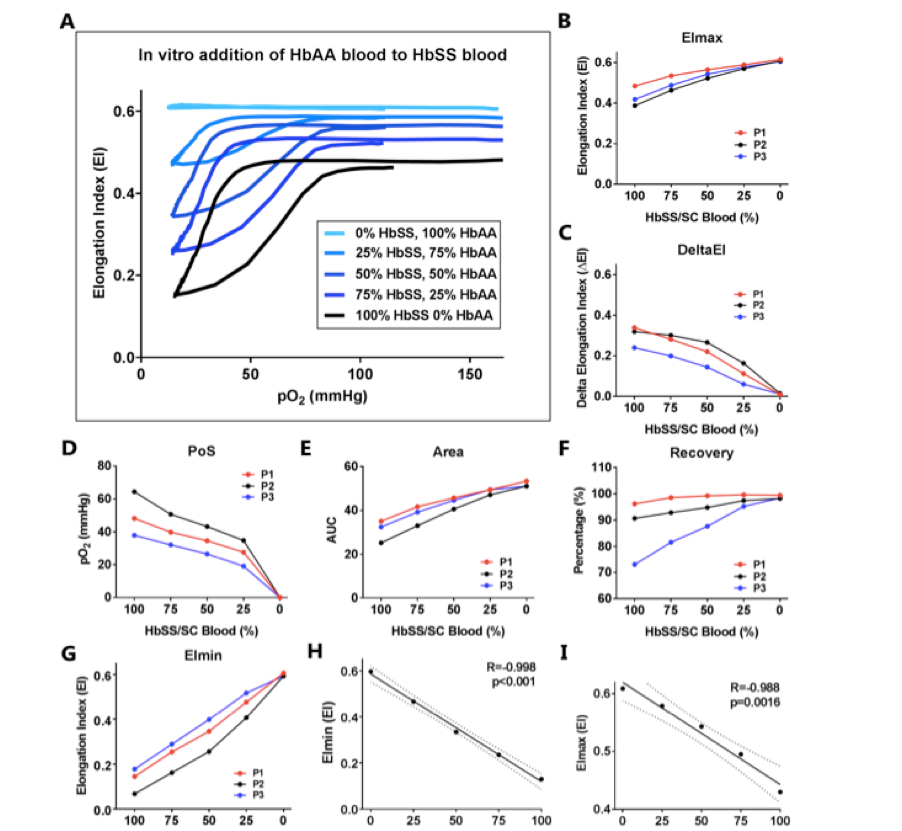
These treatments are compared to in vitro additions of HbAA blood that simulate a transfusion and a similar pattern of reduction of DeltaEI and increase in EImin are shown as the percentage of HbAA increase
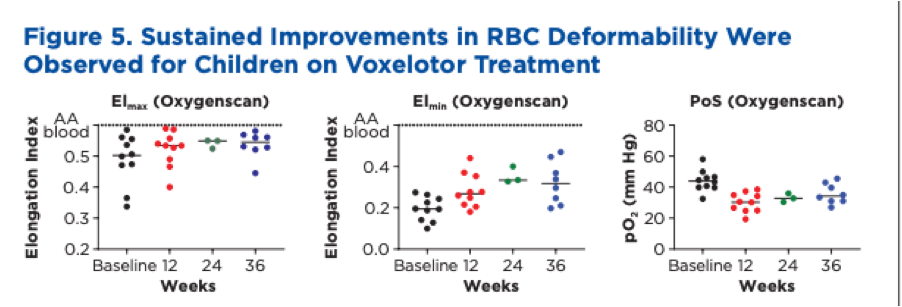
Another example of oxygenscan data from a trial comes from Global Blood Therapeutics poster presentation at ASH 2019 with data from 10 children aged 4-11 with SCD. The poster includes individual oxygenscan graphs for each patient and a summary shown below
Patients had significant increases in both EImin and EImax with DeltaEI decreasing similar to the pattern shown in the in vitro data above. There was also a significant improvement in PoS.
Etavopivat and SCD Deformability
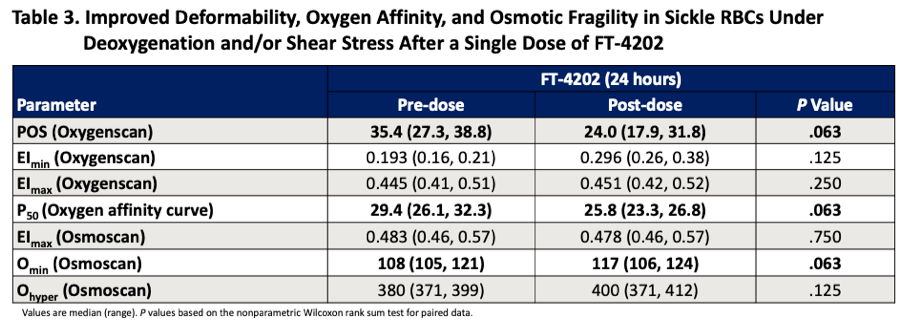
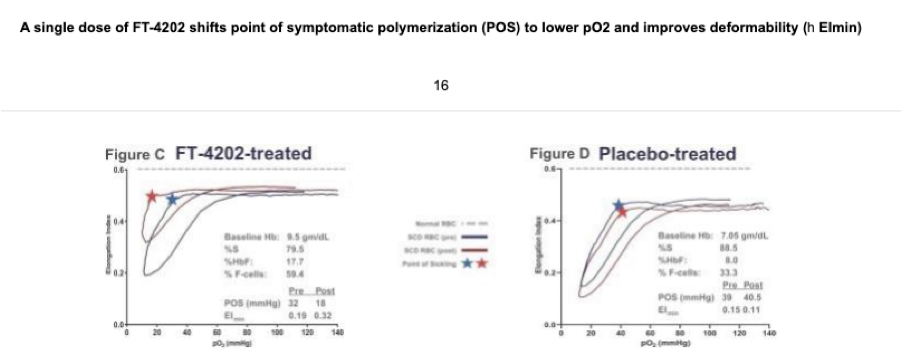
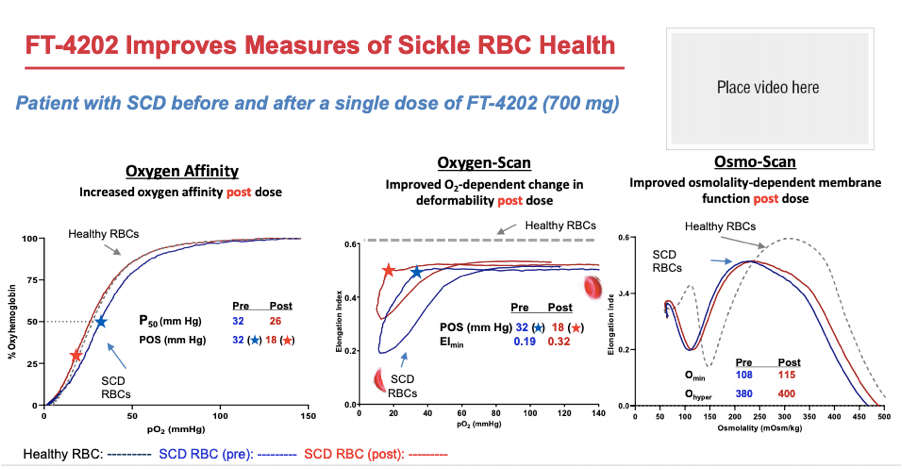
Returning back to oxygenscan data presented by Forma, the company provided initial data from the single dose portion of their Phase 1 trial at EHA 2020. In their poster presentation they provided an oxygenscan graph from a patient that received a single dose of 700mg as well as a table summarizing data from entire n=7 cohort of SCD patients that received the 700mg single dose.
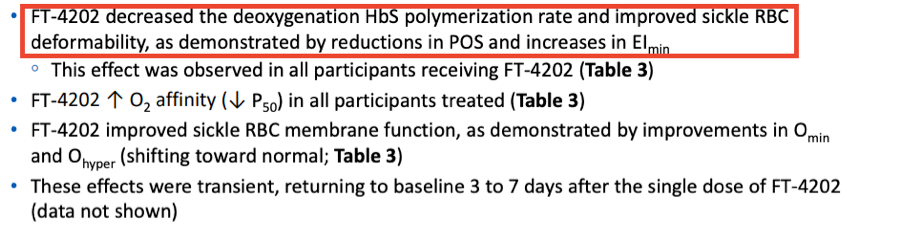
In the above table a non-significant increase in EImin and EImax are shown as well as a non-significant increase in PoS. Highlighted in the below from the results section of the poster the company states that improved sickle RBC deformability is demonstrated by reductions in PoS and increases in EImin.
This data is also included in the 10-K filing from 3/30/2021 where the company again states that deformability is improved based on EImin.
Additionally, it is included in their ASH 2020 presentation, again highlighting this single patient from the 700mg single dose cohort and presenting PoS and EImin as the measures indicating improvements in deformability
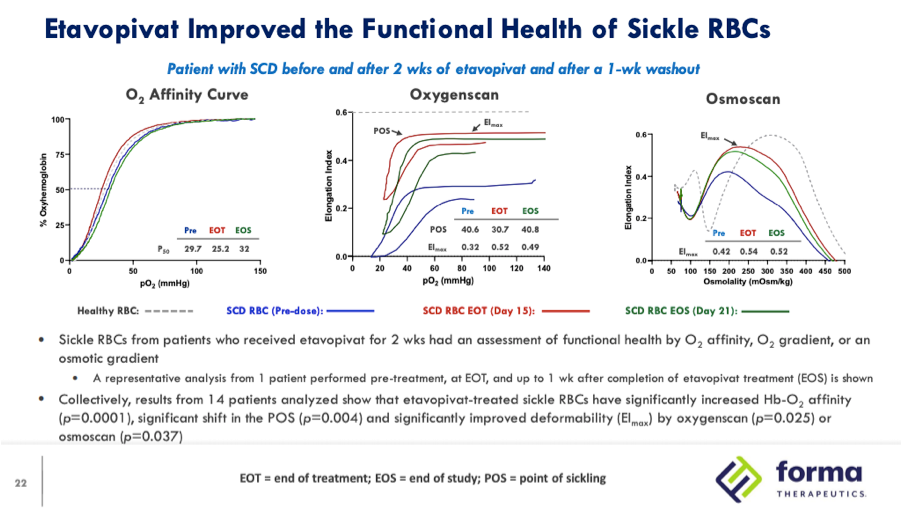
In their EHA 2021 presentation the company provides new oxygenscan data from a patient that received 300mg QD in the 14 day multiple dose portion of the Phase 1 trial:
In this slide EImin is not mentioned and instead EImax is highlighted as the measure that demonstrates improved deformability. While there is improvement in EImin the selected patient’s RBCs the graph shows an EImin pre-dose of 0 which appears atypical based on the previous examples provided by the company as well as other studies using this technology. In their Phase 1 single dose study the EImin for the 7 SCD patients at baseline ranged from .16 to .21 with a mean of .193 and in Global Blood Therapeutics’ study highlighted above the baseline mean was .19 with none of the ten patients have a baseline value of 0.
While Forma disclosed the oxygenscan graph from only one patient the last bullet point on the slide mentions that results from the 14 patients in the 300mg and 600mg QD cohorts collectively showed significantly improved deformability (EImax) by oxyegnescan (p=0.025) while there is again no mention of EImin. In the subsequent slide deformability is again defined by improvements in EImax, showing data from a patient who received 600mg QD and then rolled into the 400mg QD 12 week open-label extension.
In the Q&A section of the presentation the company was asked about oxygenscan and osmoscan data and indicated that it would be difficult to present the totality of data from the 14 day multiple dose and 12 week open-label extension portion of the Phase 1 trial and that analysis would be part of a later presentation or publication. As a result, investors will have to wait until a later date for additional clarity on baseline EImin in these patients and how it was affected by treatment with etavopivat.
Disclosure: The author is not a financial advisor and this article is not financial advice. The author maintains a long position in GBT at the time of writing but reserves the right to sell at any time.